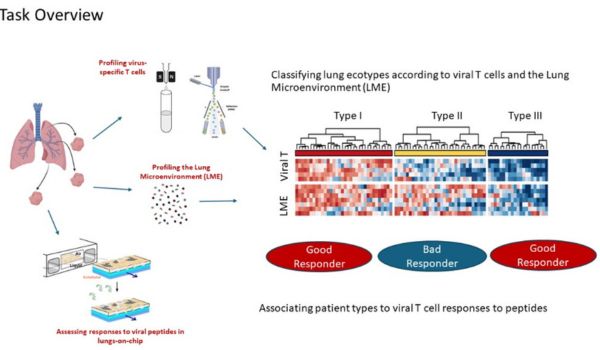
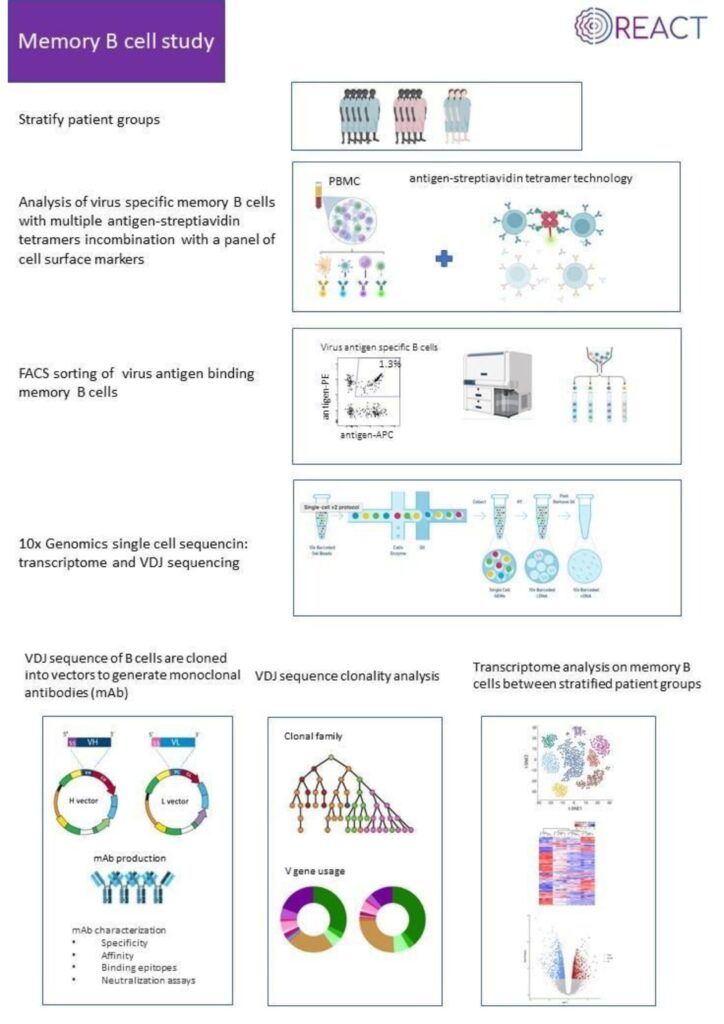
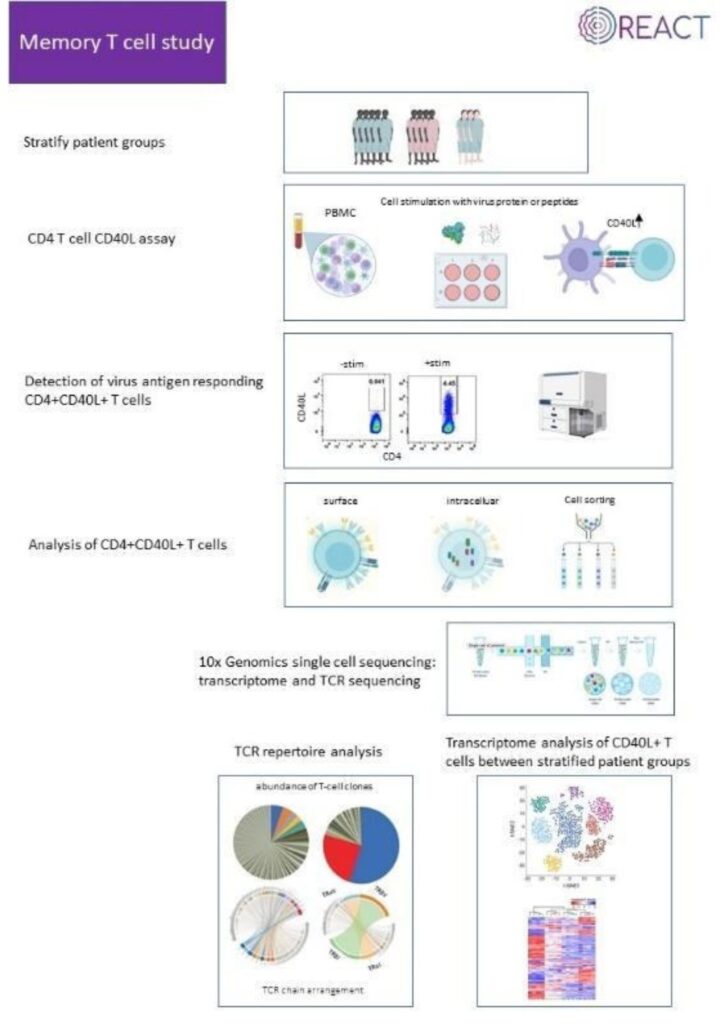
The South African researchers are all affiliated with the Faculty of Health Sciences, University of the Witwatersrand (Wits) (https://www.wits.ac.za/health/). The Wits Health Consortium (Pty) Ltd (WHC) is wholly-owned by Wits University and provides financial and human resources oversight for internationally funded research and clinical trials (https://www.witshealth.co.za/). The National Institute for Communicable Diseases (NICD) provides a strategic resource for laboratory investigation of infectious diseases of public health importance in South Africa but also the greater African continent (https://www.nicd.ac.za/). The Tiemessen Research Laboratory (Cell Biology), within the Centre for HIV and STIs at the NICD, has extensive experience in studying innate immunity, particularly in the context of HIV-1 infection. The research team has varied expertise across flow cytometry, virology, immunology, molecular biology and host genetics. The clinical researchers are based at Chris Hani Baragwanath Academic Hospital (CHBH), the third largest hospital in the world, which is a tertiary level academic hospital located in Soweto, Johannesburg (https://www.chrishanibaragwanathhospital.co.za/), and at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH). Both hospitals are affiliated with the University of the Witwatersrand and trains both undergraduate and postgraduate healthcare workers in multiple disciplines.
The Wits Health Consortium (Pty) Ltd has the infrastructure and management systems in place to provide appropriate financial and human resources oversight for internationally funded research and clinical trials. The National Institute for Communicable Diseases (NICD) provides a strategic resource for laboratory investigation of infectious diseases of public health importance in South Africa but also the greater African continent. The Institute houses several referral laboratories offering specialist laboratory testing serving surveillance and outbreak detection and management. The NICD provides routine reports and analyses, epidemic modelling, advisories, surveillance and research related to COVID-19. The Tiemessen Research Laboratory (Cell Biology), within the Centre for HIV and STIs at the NICD, has extensive experience in studying innate immunity, particularly in the context of HIV-1 infection. The research team has varied expertise across flow cytometry, virology, immunology, molecular biology and host genetics. Chris Hani Baragwanath Academic Hospital (CHBH) is a tertiary level academic hospital located in Soweto, Johannesburg, South Africa. It serves the surrounding predominantly black African population of approximately 1,8 million individuals. Many parts of Soweto rank among the poorest in Johannesburg, although individual townships tend to have a mix of wealthier and poorer residents. It remains the third largest hospital in the world, with 3 200 inpatient beds and receives referrals from surrounding areas as far as 150 km away. It is affiliated with the University of the Witwatersrand (Wits) and trains both undergraduate and postgraduate healthcare workers in multiple disciplines. The Department of Internal Medicine at Wits occupies approximately 700 of these beds. At the peaks of differing SARS-CoV-2 waves, 400 to 500 of the beds allocated to the Department of Internal Medicine were re-allocated to the care of COVID-19 patients. As inpatient numbers increase and decrease between peaks, the numbers of allocated beds follow. The COVID-19 wards remain the responsibility primarily of the Department of Internal Medicine, with assistance from other specialties when required, depending on inpatient numbers. Two of the co-PIs involved in the setup of the cohort study, which is central to the present proposal, were tasked with setting up the wards and all processes within the COVID-19 wards.
Within the National Institute for Communicable Diseases (NICD), the Tiemessen Research Laboratory (Cell Biology) leads WP7 the WHC (Cell Biology) will be responsible for coordinating with clinicians at CHBH for the recruitment of hospitalized COVID-19 patients. The clinicians involved in the study are responsible for patient care, the clinical database and analysis of clinical data. Samples are transported to the NICD, where they will be processed in the Cell Biology laboratory (Centre for HIV and STIs) and where all laboratory assays (all biomarkers, targeted DNA sequencing, genotyping assays) under Tasks 7.2 – 7.4 will be conducted, and data analysed. The whole genome RNA sequencing (single-cell and bulk neutrophils) will be done at the NICD Core Sequencing facility (Task 7.3). Human whole genome sequencing will be conducted at the SAMRC BGI Genomics Centre, Cape Town, South Africa (Task 7.4). Bioinformatics analyses of WGS are done in collaboration with the Sydney Brenner Institute for Molecular Biosciences (SBIMB), University of the Witwatersrand. WHC will be responsible for the financial oversight for studies conducted under WP7 in South Africa. WHC will collaborate with other WP participants – will ensure shipment of samples for viral/immunological analyses, sharing of data generated on WP7, analysis of integrated data and publication/dissemination of findings.